Aortic Pulse Wave Velocity in Infants
Very little is known about aortic Pulse Wave Velocity (aPWV) in childhood and infancy.
The current base of knowledge of childhood aPWV has been growing rapidly during the recent years due to the ongoing research in this area, also driven by the recent availability of operator independent non-invasive modalities such as the VICORDER®. This instrument has been used in several larger studies, in particular in pediatric nephrology [1]. aPWV, a strong predictor of mortality in various diseases in adulthood, such as end stage renal disease [2] may play an important role in predicting cardiac morbidity also in children, offering valuable assistance in optimizing and following therapy. Pediatric patients with kidney disease impress with a significantly higher aPWV when compared to healthy children [3]. In an official AHA statement a working group of paediatricians concluded “[PWV]…is recognized as likely the best clinical measure of stiffness over an arterial segment” [4]. Several authors validated Vicorder® for the use in children [5, 6] as well as collected and issued normative data [7]
In 2007 Koudsi et al. [8] presented the first report about aPWV measurement in newborns. Pulse waves in the root of the subclavian artery and the abdominal aorta were taken simultaneously. While the subclavian pulse wave was determined by an infrared probe, the abdominal wave was detected by Doppler ultrasound employing a 4 MHz probe. Waves were recorded simultaneously by a non-commercial research device. Evaluation of resulting pulse transit time and aPWV was performed offline. As tests were carried out by an experienced pediatrician, authors reported a very good intra-subject reproducibility of aPWV in their method. aPWV in the group of healthy newborns, tested not earlier than 24 hours after birth, was 4.6 m/s similar to those found in younger and school-age children.
First oscillometric PWV studies in newborns
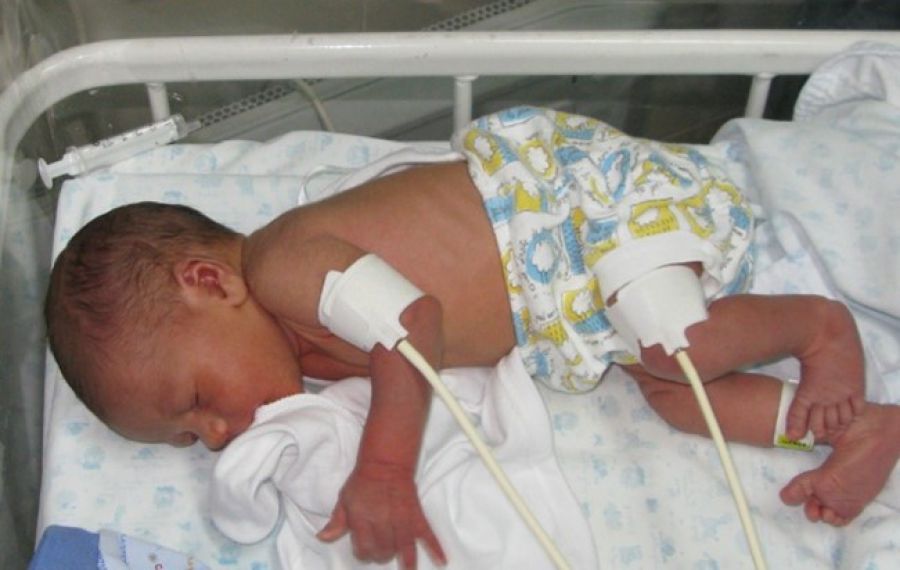
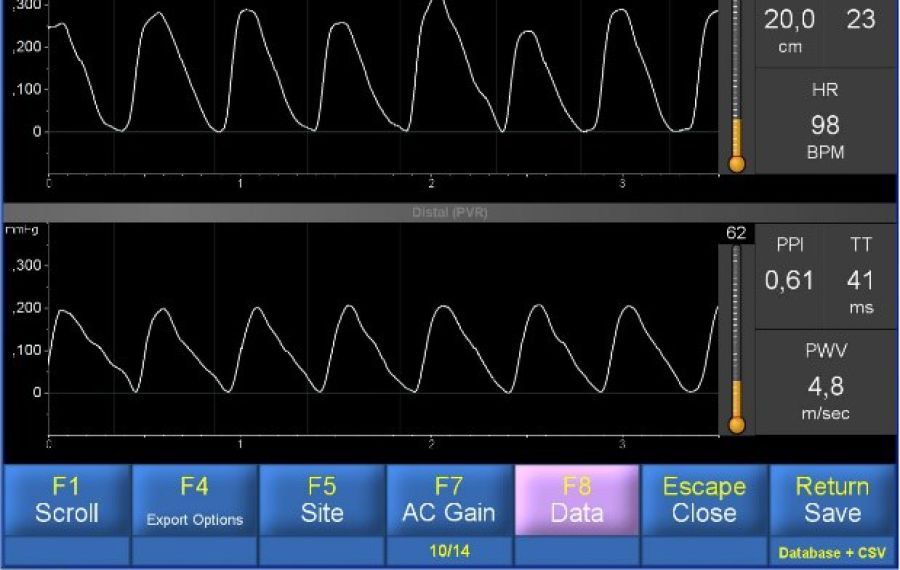
Already in 2010 we reported in a White Paper [9], that Vicorder® can be applied in neonates for determination of brachial-femoral pulse wave transit time (TT) and brachial-femoral Pulse Wave Velocity (bfPWV), the latter a recognized close estimate of aPWV. Semi-disposable digital cuffs, 2.5 cm wide, were attached to the babies right upper arm and the upper thigh. Fig. 1 shows a baby with cuffs, connected to the Vicorder® instrument tubing. Cuffs were inflated to a target pressure of 65 mmHg. Pulse traces were recorded and documented as seen below. A TT of 41 ms was observed corresponding to a aPWV of 4.8 m/s at an assumed path length of 20 cms. The child was awake and not sedated or otherwise medicated but fairly quiet, with a heart rate of 98 bpm only. As seen from Fig. 2, 23 beats were recorded in this test session which took less than 1 minute. The complete PWV test procedure including undressing the child, cuff placement, connection of tubing, and wave recording needed less than 10 minutes. Scrolling of waves revealed a stable TT reading despite intermittent movements of the child. The test was done in the presence of several gynecologists as part of a regular cardiac exam under knowledge and consent of the parents.
To the best of our knowledge this was the first time, aPWV was measured using a commercial device, in a simultaneous oscillometric mode, in real-time, yielding instantaneous results.
In about the same setting as described above, Lassalle et al. [12] reported about the application of the Vicorder® instrument in neonates in 2020. She obtained satisfactory bfPWV measurements in all five babies that she investigated. Results were comparable to the observations obtained earlier.
Special considerations in infant PWV testing
Vicorder® can be used in the routine determination of aPWV in infants, including newborns, within a very short testing time, with no inconvenience to the patient or test subject, requiring no special expertise in testing, offering operator independence and good repeatability.
In infants, central and brachial waves have a very similar contour [10]. Therefore it seems plausible that pulse waves recorded at a upper brachial site are similar to those at a central site. In contrast to mid aged and senior adults where peripheral obstruction may occur and influence brachial, axillar or femoral pulse wave propagation, this phenomenon may not be seen in infants and children.
Good correlation between brachial-femoral PWV (Vicorder®) and carotid-femoral PWV (SphygmoCor) had been reported in adults [11]. Although direct carotid-femoral aPWV is still the recommended testing procedure for aPWV in adults, the more convenient brachial-femoral test may become a standard in infant testing.
The path length measurement requires further consideration. A standardized method to estimate length may be to measure the distance between the suprasternal notch and the umbilicus for bfPWV in infants.
Instead of the above described testing in pulse volume recording (PVR) mode, TT can also be measured in the Vicorder® photo-pulse-plethysmographic (PPG) mode. In the UK, scientists attached PPG sensors to the foot and hand of a baby for recording of pulse traces. The Vicorder® PWV protocol can be switched to PPG mode allowing the synchronous display of waves and online evaluation of TT and PWV. It seems though, that the PPG traces are not as stable as the PVR waves which have a better signal to noise ratio. Further, brachial and femoral sites seem to be more appropriate for testing of Arterial Stiffness of the large vessels.
Both oscillometric PVR and PPG mode can also be used to measure TT and PWV between any two accessible locations of the infant, child, and adult body.
We conclude that Vicorder® is a simple, work and cost-efficient tool in determining PWV in infants.